Virtual nanolaboratory
Ivan Štich, Institute of Physics SAS, Bratislava
We are surrounded by many different materials in our daily lives, including metal, plastic, wood, etc. We observe these materials on length scales ranging from centimeters to meters. There is also another type of materials engineering, which takes place on much smaller, so called nanometer scales, 10-9 m, i. e. atomic scales. Nowadays, atomic scales are routinely accessible using special types of microscopes, such as the atomic force microscope, which measures the forces acting between the tip of the microscope and the sample. This microscope can also manipulate the charge of the individual atoms and molecules on the surface by injecting charge with single electron precision.

In parallel to the laboratory atomic-scale experiment, it is also necessary computer simulations. These simulations are often needed to uncover the physical and chemical processes that took place. Due to the fact that the microscope operates at the atomic scale, the virtual nanolaboratory must solve fundamental equations of the atomic world, i.e. Schrödinger’s equation, which describes the motion of electrons, as one of the main building blocks of atoms. This is usually very complicated and can only be performed using supercomputers with PFLOPs capabilities (millions of trillions of operations per second).
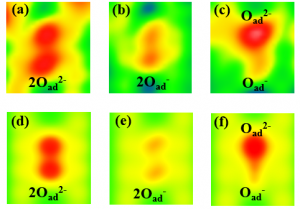
Let us discuss an example of such a laboratory experiment and its simulation. Fig. 1 shows experimental images produced by an atomic force microscope. Depicted are atoms of oxygen, which were deposited on a TiO2 surface, where they can create atom pairs. On this surface, it is favorable for the oxygen atoms to accept two electrons, i.e. to create a pair of doubly charged atoms, O2–O2-, Fig. 1 (a), that have lower energy than the singly charged atoms, O–O-, Fig. 2 (d). These energies are the outcome of computer modelling and to determine them experimentally is not easy. The atomic force microscope can be used to manipulate the charges of the oxygen atoms and create other artificial atom pairs, such as singly charged oxygen atoms, O–O-, Fig. 1 (b), or their combinations O2–O-, Fig. 1 (c), both of which have much higher energies, Fig. 2 (d).
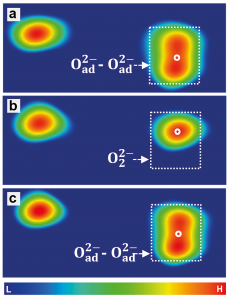
Computer modeling also allows us to verify this interpretation via a direct simulation of the images of the differently charged oxygen atoms in the atomic force microscope, Fig. 1 (d – f) and compare them to the experiment, Fig. 1 (a-c). As shown in Fig. 2, along with modifying the charge, the atomic force microscope can also manipulate, i.e. create and disrupt the chemical bonds between the atoms on the surface. If the microscope removes two electrons from the pair of doubly charged atoms, O2–O2-, it forces them to (reversibly) create a chemical bond and combine into a peroxide molecule, O22-, Fig. 2 (c). The fact that this interpretation is correct can once again be verified by computer simulations, Fig. 2 (d). If we use the microscope to remove two electrons from the O2–O2- pair and create the O–O- pair, it is energetically favorable for this pair of atoms to transform into the peroxide molecule, Fig. 2 (d). Conversely, by injecting two electrons to the peroxide molecule an O24- molecule is created which is unstable, and immediately transforms to an O2–O2- pair. Such manipulations open a way to chemical reactions fully controlled by the charge manipulations with the atomic force microscope.
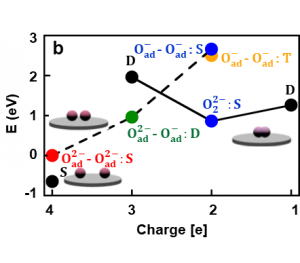
Fig. 1. Oxygen atoms in various charge states on the TiO2 surface. Images acquired experimentally from the atomic force microscope (a-c), and using computer simulations (d-f).
Fig. 2. Reversible manipulation of charged oxygen atoms into a peroxide molecule (a-c). Energies calculated using computer simulations (d). Arrows show reversible manipulation processes that result in the reaction of atoms into a molecule.

