Activation of human transglutaminases
Project name: GLIKOBIO, Project Leader: Attila Fekete
Home inst.: the Division of Clinical Laboratory Science, the Faculty of Medicine, the University of Debrecen, Debrecen, Hungary
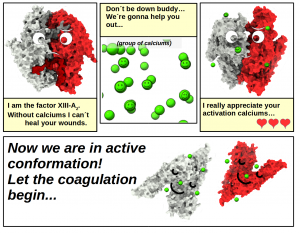
Probably everyone has seen or had a bleeding wound. The process of our blood turning from a liquid to a solid (coagulation) to stop the leak is a complex process involving a cascade of chemical reactions. The A subunits of factor XIII of the blood coagulation cascade (FXIII-A2) play an important role in this process (crosslinking γ-fibrins), and as well as tissue transgluatminase (TG2), they also play a key role in homeostasis. Both of these enzymes are human transglutaminases (TGases), part of an experimentally and structurally well studied system. Though their biological roles are quite different, for both TGases activation only occurs in the presence of calcium ions (Ca2+). Dysfunction in this process can lead to diseases such as Celiac.
Attila Fekete and colleagues use microsecond long classical all-atom molecular dynamics simulations in order to clarify the early events of their calcium induced activation. These kinds of simulations are quite useful when we want to see something that is normally invisible to other techniques. Furthermore we can easily get an insight into the time-dependent dynamic nature of biomacromolecules. These computer (or “in silico”) experiments require enormous amounts of computer power to handle all the information and calculation of the Newtonian laws simultaneously for a system which contains thousands of water molecules, some ions, and of course the protein itself. Hence supercomputers are required.
Both of the studied proteins have at least two different structures, namely they can take on inactive (closed) or active (open) conformations. It was thought that TG2 could bind up to six calcium ions, but only five of them had been identified. Attila and colleagues’ results suggest that three of the five sites can indeed bind calcium, but the other two sites do not bind Ca2+ at all. They discovered at least three previously unknown binding sites, one of which is probably the mysterious sixth binding site.